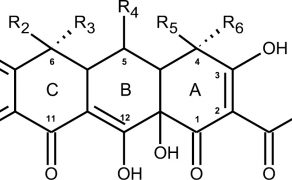
Charakterystyka tetracyklin stosowanych w medycynie weterynaryjnej
Piśmiennictwo
- Nelson M.L., Levy S.B.: The history of the tetracyclines. „Ann. N. Y. Acad. Sci.”, 2011 vol. 1241, s. 17-32.
- Charest M.G. i wsp.: Synthesis of minus-tetracycline. „J. Am. Chem. Soc.”, 2005, vol. 127, s. 8292-8293.
- Chopra I., Roberts M.: Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. „Microbiol. Mol. Biol. Rev.”, 2001, vol. 65, s. 232-260.
- McCormick J.R. i wsp.: A new family of antibiotics: the demethyltetracyclines. „J. Am. Chem. Soc.”, 1957, vol. 79, s. 4561-4563.
- Dürckheimer W.: Tetracyclines: chemistry, biochemistry, and structure-activity relations. „Angew. Chem. Internal. Ed. Engl.”, 1975, vol. 14, s. 721-734.
- Van Bambeke F., Balzi E., Tulkens P.M.: Antibiotic efflux pumps. „Biochem. Pharmacol.”, 2000, vol. 60, s. 457-470.
- Miyake Y. i wsp.: In vitro activity of tetracyclines, macrolides, quinolones, clindamycin and metronidazole against periodontopathic bacteria. „J. Periodont. Res.”, 1995, vol. 30, s. 290-293.
- Zhanel G.G. i wsp.: The glycylcyclines: a comparative review with the tetracyclines. „Drugs”, 2004, vol. 64, s. 63-88.
- Dahl E.L. i wsp.: Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. „Antimicrob. Agents. Chemother.”, 2006, vol. 50, s. 3124-3131.
- Dibner J.J., Richards J.D.: Antibiotic growth promoters in agriculture: history and mode of action. „Poult. Sci”, 2005, vol. 84, s. 634-643.
- Klein N.C., Cunha B.A.: Tetracyclines. „Med. Clin. North. Am.”, 1995, vol. 79, s. 789-801.
- Thaker M., Spanogiannopoulos P., Wright G.D.: The tetracycline resistome. „Cell. Mol. Life. Sci.”, 2010, vol. 67, s. 419-431.
- Petkovic H. i wsp.: Genetics of Streptomyces rimosus, the oxytetracycline producer. „Microbiol. Mol. Biol. Rev.”, 2006, vol. 70, s. 704-728.
- Moore I.F., Hughes D.W., Wright G.D.: Tigecycline is modified by the flavin-dependent monooxygenase Tetx. „Biochemistry”, 2005, vol. 44, s. 11829-11835.
- Epe B., Woolley P.: The Binding of 6-demethylchlortetracycline to 70S, 50S and 30S ribosomal particles: a quantitative study by fluorescence anisotropy. „EMBO J.”, 1984, vol. 3, s. 121-126.
- Goldman R.A. i wsp.: Photoincorporation of tetracycline into Escherichia coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. „Biochemistry”, 1983, vol. 22, s. 359-368.
- Brodersen D.E. i wsp.: The structural basis for the action of the antibiotics tetracycline, pactamycin and hygromycin B on the 30S ribosomal subunit. „Cell”, 2000, vol. 103, s. 1143-1154.
- Schnappinger D., Hillen W.: Tetracyclines: antibiotic action, uptake, and resistance mechanisms. „Arch. Microbiol.”, 1996, vol. 165, s. 359-369.
- Griffin M.O., Ceballos G., Villarreal F.J.: Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. „Pharmacol. Res.”, 2011, vol. 63, s 102-107.
- Acharya M.R. i wsp.: Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. „Drug. Resist. Updat.”, 2004, vol. 7, s. 195-208.
- Hu J. i wsp.: Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. „Nat. Rev. Drug. Discov.”, 2007, vol. 6, s. 480-498.
- Chen M. i wsp.: Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of huntington disease. „Nat. Med.”, 2000, vol. 6, s. 797-801.
- Amin A.R. i wsp.: A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. „Proc. Natl. Acad. Sci. USA”, 1996, vol. 93, s. 14014-14019.
- Kraus R.L. i wsp.: Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. „J. Neurochem.”, 2005, vol. 94, s. 19-827.
- Krakauer T., Buckley M.: Doxycycline is anti-inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. „Antimicrob. Agents Chemother.”, 2003, vol. 47, s. 3630-3633.
- Riond J.L. i wsp.: Cardiovascular effects and fatalities associated with intravenous administration of doxycycline to horses and ponies. „Equine Vet. J.”, 1992, vol. 24, s. 41-45.
- Pollet R.A. i wsp.: Pharmacokinetics of chlortetracycline potentiation with citric acid in the chicken. „Am. J. Vet. Res.”, 1983, vol. 44, s. 1718-1721.
- Anadón A. i wsp.: Plasma disposition and tissue depletion of chlortetracycline in the food producing animals, chickens for fattening. „Food Chem. Toxicol.”, 2012, vol. 50, s. 2714-2721.
- Pollet R.A., Glatz C.E., Dyer D.C.: Oral absorption of chlortetracycline in turkeys: influence of citric acid and Pasteurella multocida infection. „Poult. Sci.”, 1984, vol. 63, s. 1110-1114.
- Dyer D.C.: Pharmacokinetics of chlortetracycline in the turkey: evaluation of biliary secretion. „Am. J. Vet. Res.”, 1988, vol. 49, s. 36-37.
- Nielsen P., Gyrd-Hansen N.: Bioavailability of oxytetracycline, tetracycline and chlortetracycline after oral administration to fed and fasted pigs. „J. Vet. Pharmacol. Ther.”, 1996, vol. 19, s. 305-311.
- Kilroy C.R. i wsp.: Chlortetracycline in swine-bioavailability and pharmacokinetics in fasted and fed pigs. „J. Vet. Pharmacol. Ther.”, 1990, vol. 13, s. 49-58.
- Wanner M. i wsp.: Influence of dietary citric acid and calcium on the bioavailability of orally administered chlortetracycline in piglets. „J. Vet. Med. A.”, 1991, vol. 38, s. 755-762.
- Mevius D.J., Vellenga L., Breukink H.J.: Pharmacokinetics and renal clearance of oxytetracycline in piglets following intravenous and oral administration. „Vet. Quarter”, 1986, vol. 8, s. 74-85.
- Pijpers A. i wsp.: The influence of disease on feed and water consumption and on pharmacokinetics of orally administered oxytetracycline in pigs. „J. Anim. Sci.”, 1991, vol. 69, s. 2947-2954.
- Nouws J.F., Vree T.B.: Effect of injection site on the bioavailability of an oxytetracycline formulation in ruminant calves. „Vet. Quarter”, 1983, vol. 5, s. 165-170.
- Nouws J.F., Van Ginneken C.A., Ziv G.: Age-dependent pharmacokinetics of oxytetracycline in ruminants. „J. Vet. Pharmacol. Ther.”, 1983, vol. 6, s. 59-66.
- Grondel J.L. i wsp.: Pharmacokinetics and tissue distribution of oxytetracycline in carp, Cyprinus Carpio L., following different routes of administration. „J. Fish. Dis.”, 1987, vol. 10, s. 153-163.
- Elema M.O., Hoff K.A., Kristensen H.G.: Bioavailability of oxytetracycline from medicated feed administered to atlantic salmon (Salmo Salar L.) in seawater. „Aquaculture”, 1996, vol. 143, s. 7-14.
- Grondel J.L. i wsp.: Comparative pharamockinetics of oxytetracycline in rainbow trout (Salmo gairdneri) and african catfish (Clarias gariepinus). „J. Vet. Pharmacol. Ther.”, 1989, vol. 12, s. 157-162.
- Anadón A.: Pharmacokinetics of tetracycline in chickens after intravenous administration. „Poult. Sci.”, 1985, vol. 64, s. 2273-2279.
- Kniffen T.S. i wsp.: Bioavailability, pharmacokinetics, and plasma concentration of tetracycline hydrochloride fed to swine. „Am. J. Vet. Res.”, 1989, vol. 50, s. 518-521.
- Rajaian H., Soleimani Mohammadi E.: Pharmacokinetics of tetracycline hydrochloride in fat-tailed sheep. „IJVR”, 2007, vol. 8, s. 138-143.
- Plakas S.M., Mcphearson R.M., Guarino A.M.: Disposition and bioavailability of 3H-tetracycline in the channel catfish (Ictalurus punctatus). „Xenobiotica”, 1988, vol. 18, s. 83-93.
- Anadón A. i wsp.: Pharmacokinetics of doxycycline in broiler chickens. „Avian Pathol.”, 1994, vol. 23, s. 79-90.
- 46. Laczay P. i wsp.: Pharmacokinetics and bioavailability of doxycycline in fasted and nonfasted broiler chickens. „Acta Vet. Hung.”, 2001, vol. 49, s. 31-37.
- Santos M.D. i wsp. Pharmacokinetics and bioavailability of doxycycline in turkeys. „J. Vet. Pharmacol. Ther.”, 1996, vol. 19, s. 274-280.
- Baert K. i wsp.: Pharmacokinetics and oral bioavailability of a doxycycline formulation (Doxycycline 75%) in nonfasted young pigs. „J. Vet. Pharmacol. Ther.”, 2000, vol. 23, s. 45-48.
- He J. i wsp.: Pharmacokinetics of doxycycline hydrochloride sustain-released injection in swine. „Chin. J. Vet. Sci.”, 2008, vol. 28, s. 175-180.
- Riond J.L., Riviere J.E.: Pharmacokinetics and metabolic inertness of doxycycline in young pigs. „Am. J. Vet. Res.”, 1990, vol. 51, s. 1271-1275.
- Meijer L.A. i wsp.: Pharmacokinetics and bioavailability of doxycycline hyclate after oral administration in calves. „Vet Quarter”, 1993, vol. 15, s. 1-5.
- Riond J.L., Tyczkowska K., Riviere J.E.: Pharmacokinetics and metabolic inertness of doxycycline in calves with mature or immature rumen function. „Am. J. Vet. Res.”, 1989, vol. 50, s. 1329-1333.
- Gutiérrez L. i wsp.: Pharmacokinetics of an injectable long-acting formulation of doxycycline hyclate in dogs. „Acta. Vet. Scand.”, 2012, vol. 54, s. 1-7.
- Riond J.L., Vaden S.L., Riviere J.E.: Comparative pharmacokinetics of doxycycline in cats and dogs. „J. Vet. Pharmacol. Ther.”, 1990, vol. 13, s. 415-424.
- Wilson R.C. i wsp.: Pharmacokinetics of doxycycline in dogs. „Can. J. Vet. Res.”, 1988, vol. 52, s. 12-14.
- Gabler W.L.: Fluxes and accumulation of tetracyclines by human blood cells. „Res. Commun. Chem. Pathol. Pharmacol.”, 1991, vol. 72, s. 39-51.
- Davis J.L., Salmon J.H., Papich M.G.: Pharmacokinetics and tissue distribution of doxycycline after oral administration of single and multiple doses in horses. „Am. J. Vet. Res.”, 2006, vol. 67, s. 310-316.
- Harms C.A. i wsp.: Pharmacokinetics of oxytetracycline in loggerhead sea turtles (Caretta caretta) after single intravenous and intramuscular injections. „J. Zoo Wildl. Med.”, 2004, vol. 35, s. 477-488.
- Ames T.R., Larson L.V., Stowf C.M.: Oxytetracycline concentrations in healthy and diseased calves. „Am. J. Vet. Res.”, 1983, vol. 44, s. 1354-1357.
- Oka H., Ito Y., Matsumoto H.: Chromatographic analysis of tetracycline antibiotics in foods. „J. Chromatogr. A.”, 2000, vol. 882, s. 109-133.
- Croubels S. i wsp.: Liquid chromatographic separation of doxycycline and 4-epidoxycycline in a tissue depletion study of doxycycline in turkeys. „J. Chromatogr. B. Biomed. Sci. Appl.”, 1998, vol. 708, s. 145-152.
- Zurhelle G. i wsp.: Metabolites of oxytetracycline, tetracycline, and chlortetracycline and their distribution in egg white, egg yolk, and hen plasma. „J. Agric. Food. Chem.”, 2000, vol. 48, s. 6392-6396.
- Cherlet M., De Baere S., De Backer P.: Quantitative analysis of oxytetracycline and its 4-epimer in calf tissues by high-performance liquid chromatography combined with positive electrospray ionization mass spectrometry. „Analyst”, 2003, vol. 128, s. 871-878.
- Schach von Wittenau M., Twomey T.M.: The disposition of doxycycline by man and dog. „Chemotherapy”, 1971, vol. 16, s. 217-228.
- Anonymous: Tetracylines veterinary – systemic. „J. Vet. Pharmacol. Ther.”, 2003, vol. 26 suppl 2, s. 225-252.
- Humbert P. i wsp.: Use of anti-collagenase properties of doxycycline in treatment of alpha 1-antitrypsin deficiency panniculitis. „Acta Derm. Venereol.”, 1991, vol. 71, s. 189-194.
- Joshi R.K. i wsp.: Successful treatment of Sweet’s syndrome with doxycycline. „Br. J. Dermatol.”, 1993, vol. 128, s. 584-586.
- Bachelez H. i wsp.: The use of tetracyclines for the treatment of sarcoidosis. „Arch. Dermatol.”, 2001, vol. 137, s. 69-73.
- Berth-Jones J. i wsp.: The successful use of minocycline in pyoderma gangrenosum – a report of seven cases and review of the literature. „J. Dermatol. Treat.”, 1989, vol. 1, s. 23-25.
- Tilley B.C. i wsp.: Minocycline in rheumatoid arthritis: a 48 week double-blind placebo controlled trial. „Ann. Intern. Med.”, 1995, vol. 122, s. 81-89.
- Le C.H., Morales A., Trentham D.E.: Minocycline in early diffuse scleroderma. „Lancet”, 1998, vol. 352, s. 1755-1756.
- Hidalgo M., Eckhardt S.G.: Development of matrix metalloproteinase inhibitors in cancer therapy. „J. Natl. Cancer. Inst.”, 2001, vol. 93, s. 178-193.
- Ramamurthy N.S. i wsp.: Inhibition of matrix metalloproteinase-mediated periodontal bone loss in rats: a comparison of 6 chemically modified tetracyclines. „J. Periodontol.”, 2002, vol. 73, s. 726-734.
- Weinberg E.D.: The mutual effects of antimicrobial compounds and metallic cations. „Bacteriol. Rev.”, 1957, vol. 21, s. 46-68.
- Guerra W. i wsp.: Synthesis, characterization, and antibacterial activity of three palladium (II) complexes of tetracyclines. „J. Inorg. Biochem.”, 2005, vol. 99, s. 2348-2354.
- Wessels J.M. i wsp.: The complexation of tetracycline and anhydrotetracycline with Mg2+ and Ca2+: a spectroscopic study. „J. Phys. Chem. B.”, 1998, vol. 102, s. 9323-9331.
- Nelson M.L.: Chemical and biological dynamics of tetracyclines. „Adv. Dent. Res.”, 1998, 12: 5-11.
- Mortimer P.G.S., Piddock L.J.V.: The accumulation of five antibacterial agents in porin-deficient mutants of Escherichia coli. „J. Antimicrob. Chemother.”, 1993, 32: 195-213.
- Nikaido H., Thanassi D.G.: Penetration of lipophilic agents with multiple protonation sites into bacterial cells: tetracyclines and fluoroquinolones as examples. „Antimicrob. Agents. Chemother.”, 1993, vol. 37, s. 1393-1399.
- Novák-Pékli M., Mesbah M.E.H., Pethő G.: Equilibrium studies on tetracycline-metal ion systems. „J. Pharm. Biomed. Anal.”, 1996, vol. 14, s. 1025-1029.
- Leyden J.J.: Absorption of minocycline hydrochloride and tetracycline hydrochloride, effect of food, milk, and iron. „J. Am. Acad. Dermatol.”, 1985, vol. 12, s. 308-312.
- Wouters M.F., Van Koten-Vermeulen J.E., Van Leeuwen F.X.: Chlortetracycline and tetracycline. WHO Food Additive Series 2005, vol. 36.
- Reboud A.M., Dubost S., Reboud J.P.: Photoincorporation of tetracycline into rat‐liver ribosomes and subunits. „Eur. J. Biochem.”, 1982, vol. 124, s. 389-396.
- Papich M.G.: Antimicrobial therapy for gastrointestinal diseases. „Vet. Clin. North. Am. Equine. Pract.”, 2003, vol. 19, s. 645-663.
- Carlborg B., Densert O.: Esophageal lesions caused by orally administered drugs. „Eur. Surg. Res.”, 1980, vol. 12, s. 270-282.
- German A.J. i wsp.: Oesophageal strictures in cats associated with doxycycline therapy. „J. Feline Med. Surg.”, 2005, vol. 7, s. 33-41.
- Grossman E.R., Walchek A., Freedman H.: Tetracyclines and permanent teeth: the relation between dose and tooth color. „Pediatrics”, 1971, vol. 47, s. 567-570.
- Engesaeter L.B., Underdal T., Langeland N.: Effects of oxytetracycline on mineralization of bone in young rats. „Acta Orthop. Scand.”, 1980, vol. 51, s. 459-465.
- Farombi E.O. i wsp.: Tetracycline-induced reproductive toxicity in male rats: effects of vitamin C and N-acetylcysteine. „Exp. Toxicol. Pathol.”, 2008, vol. 60, s. 77-85.
- Riond J.L., Riviere J.E.: Effects of tetracyclines on the kidney in cattle and dogs. „J. Am. Vet. Med. Assoc.”, 1989, vol. 195, s. 995-997.
- Stevenson S.: Oxytetracycline nephrotoxicosis in two dogs. „J. Am. Vet. Med. Assoc.”, 1980, vol. 176, s. 530-531.
- Vivrette S. i wsp.: Hemodialysis for treatment of oxytetracycline-induced acute renal failure in a neonatal foal. „J. Am. Vet. Med. Assoc.”, 1993, vol. 203, s. 105-107.
- Shaddad S.A. i wsp.: The effect of oxytetracycline on growth and lipid metabolism in poultry. „Comp. Biochem. Physiol. C.”, 1985, vol. 80, s. 375-380.
- Sutton T.A. i wsp.: Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. „Am. J. Physiol. Renal. Physiol.”, 2005, vol. 288, s. 91-97.
- Ward G.S., Guiry C.C., Alexander L.L.: Tetracycline-induced anaphylactic shock in a dog. „JAVMA”, 1982, vol. 180, s. 770-771.
- Hasan T. i wsp.: Mechanisms of tetracycline phototoxicity. „J. Invest. Dermatol.”, 1984, vol. 83, s. 179-183.
- Lim H.W., Novotny H., Gigli I.: Role of complement and polymorphonuclear cells in demethylchlortetracycline-induced phototoxicity. „J. Clin. Invest.”, 1983, vol. 71, s. 1326-1335.
- Gyrd-Hansen N., Rasmussen F., Smith M.: Cardiovascular effects of intravenous administration of tetracycline in cattle. „J. Vet. Pharmacol. Ther.”, 1981, vol. 4, s. 15-25.
prof. dr hab. Jerzy Jaroszewski
Katedra Farmakologii i Toksykologii
Wydział Medycyny Weterynaryjnej
Uniwersytet Warmińsko-Mazurski w Olsztynie
10-719 Olsztyn, ul. Oczapowskiego 13
Mogą zainteresować Cię również
Znajdź swoją kategorię
2626 praktycznych artykułów - 324 ekspertów - 22 kategorii tematycznych
Weterynaria w Terenie

Ostertagioza bydła – nowe aspekty epizootiologiczne
Piśmiennictwo Armour J., Jennings F.W., Murray M., Selman I.: Bovine ostertagiasis – clinical aspects, pathogenesis, epidemiology and control. [W:] Urquhart G.M., Armour J.: Helminth Diseases of Cattle, Sheep and Horses in Europe. Wyd. Robert MacLehose Ltd, Veterinary School, University of Glasgow, 1973, 11-22. Charlier J., Duchateau L., Claerebout E., Vercruysse J.: Assessment of the repeatability […]

Ostertagioza bydła – nowe aspekty epizootiologiczne
Piśmiennictwo Armour J., Jennings F.W., Murray M., Selman I.: Bovine ostertagiasis – clinical aspects, pathogenesis, epidemiology and control. [W:] Urquhart G.M., Armour J.: Helminth Diseases of Cattle, Sheep and Horses in Europe. Wyd. Robert MacLehose Ltd, Veterinary School, University of Glasgow, 1973, 11-22. Charlier J., Duchateau L., Claerebout E., Vercruysse J.: Assessment of the repeatability […]

Przypadek zapalenia mózgu i mięśnia sercowego u prosiąt ssących
Piśmiennictwo Alexandersen S., Knowles N.J., Dekker A., Belsham G.J., Zhang Z., Koenen F.: Picornaviruses. [In:] Zimmenrman J.J., D’Allaire S., Taylor D.J.: Disease of Swine. Blackwell Publishing 2012, Ames (Iowa, USA), 606-620. Bakkali Kassimi L., Gonzague M., Boutrouille A., Cruciere C.. Detection of Encephalomyocarditis virus in clinical samples by immunomagnetic separation and one-step RT-PCR. „Journal of […]

Choroby koni. Weterynaria praktyczna. Profilaktyka, rutynowe szczepienia
Publikacja stanowi fragment książki Choroby koni. Weterynaria praktyczna Wirus grypy jest wysoce zaraźliwy i szybko się rozprzestrzenia, a sama choroba niesie ze sobą bardzo poważne skutki i na długi czas wyklucza konia z udziału w zawodach czy wyścigach. Z tego względu wprowadzono wymóg obowiązkowych szczepień przeciwko grypie dla wszystkich koni wyścigowych oraz koni sportowych. Opory […]
Wskazówki na wypadek widocznego niepowodzenia terapii antybiotykowej. Kryteria skutecznej terapii oraz kluczowe pytania jako 5 kroków drzewa analitycznego
Czynniki powiązane z użyciem antybiotyku w terapii: Czy wybór antybiotyku opierał się na badaniach klinicznych i dodatkowych (diagnoza, antybiogram)? Sprawdź odpowiedź na pytanie 3. Farmakokinetyka/farmakodynamika wybranego antybiotyku? Koncentracja i czas działania antybiotyku w zakażonej tkance a efektywność w stosunku do czynnika bakteryjnego wywołującego chorobę (spektrum działania antybiotyku, wrażliwość z antybiogramu – odpowiedzi na pytanie 3.). […]

Czarno na białym – mastitis okiem praktyka – rozmowa z dr. n. wet. Sebastianem Smulskim
Rozmowa z dr. n. wet. Sebastianem Smulskim, pracownikiem Uniwersytetu Przyrodniczego w Poznaniu, specjalistą w dziedzinie profilaktyki i leczenia mastitis u krów, który w swoich badaniach zgłębia tematykę zapalenia gruczołu mlekowego u bydła, zarówno w aspekcie naukowym, jak i praktycznym. Większość zapaleń gruczołu mlekowego ma etiologię bakteryjną. Dlaczego, pomimo rozwoju mikrobiologii, medycyny weterynaryjnej i prowadzonych badań, […]

XVIII FORUM ZOOTECHNICZNO-WETERYNARYJNE: NOWE HORYZONTY W ROZRODZIE ZWIERZĄT
Na Uniwersytecie Przyrodniczym w Poznaniu w dniach 18-19 kwietnia br. odbyło się XVIII Forum Zootechniczno-Weterynaryjne pod hasłem „Rozród zwierząt w dobie selekcji genomowej”. To wydarzenie zgromadziło liczne grono lekarzy weterynarii oraz hodowców, by omówić najnowsze osiągnięcia w dziedzinie hodowli i rozrodu zwierząt. Organizacja Forum była wspólnym przedsięwzięciem Poznańskiego Koła Polskiego Towarzystwa Zootechnicznego, Wielkopolskiego Oddziału Polskiego […]