Hemofilia typu A i B u psów
Canine haemophilia A and B
Canine haemophilia A and B are the most important inherited defects of secondary haemostasis. The incidence of canine haemophilia A is about three or four times higher than haemophilia B (Brooks et al., 2003). These disorders are caused by a mutation in the canine factor VIII (FVIII) and canine FIX gene, (Brooks et al., 1997; Gu et al., 1999; Mischke, 2012; Alcaraz et al., 2013), which leads to the absence or dysfunction of FVIII and FIX (Brooks, 1999; Stokol, 2005), respectively, and results in delayed blood clotting (Mischke, 2012).
This article focuses on the role of FVIII and FIX in the coagulation system, the molecular basis of canine haemophilia A and B, as well as clinical signs and diagnosis of haemophilia A and B in dogs.
Role of FVIII and FIX in the coagulation system
The importance of FVIII and FIX in the coagulation system is illustrated by the fact that low circulating levels of FVIII or FIX complicate the assembly of the intrinsic factor Xase complex and impedes the propagation phase of the coagulation system (Mann et al., 2003), resulting in X-linked disorders known as haemophilia A and B, respectively (Stokol, 2005; Brooks, 1999).
The glycoprotein FVIII circulates in plasma as an inactive, pro-cofactor form in a non-covalent complex with von Willebrand fator (vWF) (Nichols et al., 1993; Sadler, 1998; Fay, 2004; Fay et al., 2005). The binding site on canine FVIII for vWF involves the amino acid residues 1666 to 1676 in the A3 domain of the FVIII (Cameron et al., 1998). In dogs, vWF stabilizes the structure of FVIII in plasma. However, canine FVIII does not appear to be as dependent on vWF for stabilization as human FVIII (Turecek et al., 1997; Denis et al., 1999). In contrast, FIX is a vitamin K-dependent coagulation factor (Mathur et al., 1997) that circulates as a single-chain zymogene in blood plasma (Lacobelli, 2008).
During blood coagulation, FIX activation by FVIIa-TF occurs early in the course of the fibrin formation (Osterud et al., 1977; Lawson et al., 1991) and it finishes when the FVIIa-TF complex is inhibited by the tissue factor pathway inhibitor (Monroe et al., 2007). In this situation, FXIa supplements the activation of FIX (Mann et al., 2003; Smith et al., 2008). In dogs, activation sites on FIX for FXIa have been located at Arg 146-Ala 147 and Arg 178-Val 179 in the activation peptide domain of the FIX molecule (Axelrod et al., 1990).
During the propagation of coagulation, coagulant FVIII is activated by early generated thrombin in a positive feedback loop (Lane et al., 2005). Thrombin is an extremely efficient activator of FVIII (Esmon et al., 1996). In dogs, thrombin cleaves three peptide bonds at Arg 366-Ser 367, Arg 734-Ser 735 and Arg 1681-Ser 1682 during the FVIII activation (Cameron et al., 1998).
Once both factors are activated, the FVIIIa forms a complex, known as intrinsic FXase complex (Blostein et al., 2003), with FIXa and FX on the activated platelet surface (Lacroix-Desmazes et al., 2008). Activated platelets expose binding sites for FVIIIa (Ahmad et al., 2000), FIXa (Ahmad et al., 1989) and FX (Scandura et al., 1996; Ahmad et al., 2003A). The main role of cofactor FVIIIa in this complex is to present FX to the protease enzyme FIXa (Ahmad et al., 2003B), which catalyses the proteolytic conversion of FX to FXa in the presence of calcium (Van Dieijen et al., 1981; Mathur et al., 1997). In addition, the intrinsic FXase complex has been considered to be responsible for most of the FXa produced during the coagulation cascade (Mann et al., 2003), being critical for the propagation phase of coagulation at the site of vascular injury (Fay, 2004).
Known mutation of canine FVIII and FIX gene
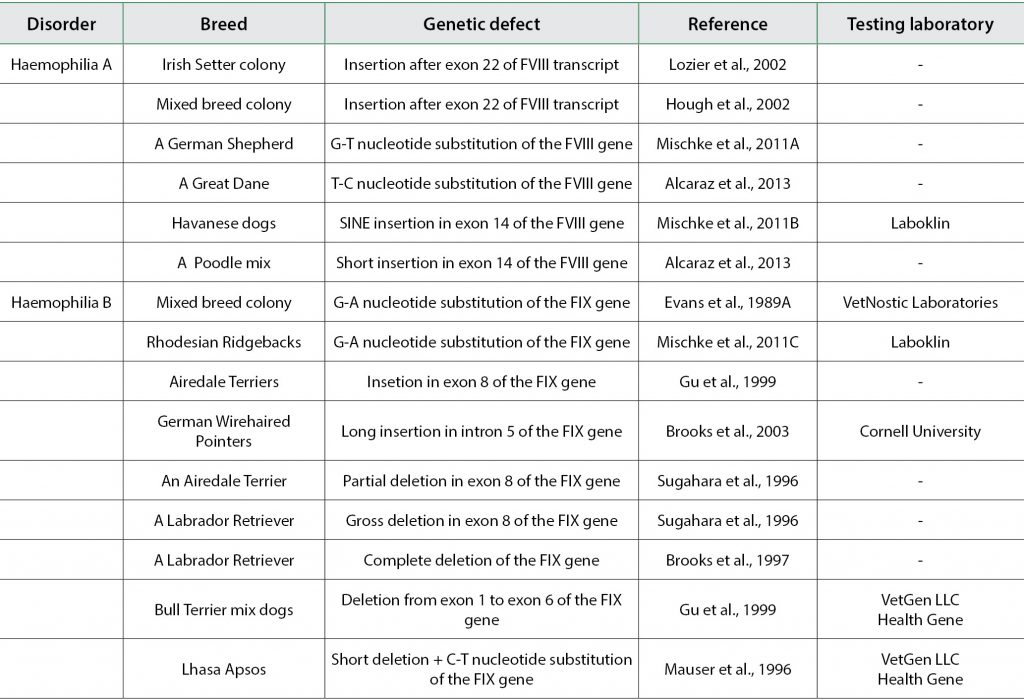
The molecular basis of haemophilia A and B are diverse in humans (Bowen, 2002) and in dogs (Sugahara et al., 1996; Brooks et al., 1997; Gu et al., 1999; Mischke, 2012; Alcaraz et al., 2013), i.e. the phenotype of both disorders is caused by mutational heterogeneity between the breeds and even within breeds.
The molecular defect causing haemophilia A has been identified in five different dog breeds and in mixed dogs (Tab. 1). The first described mutation in the canine FVIII gene was observed in a haemophilic Irish Setter colony at the University of North Carolina. Severe haemophilic dogs showed prolonged coagulation parameters and a lack of FVIII activity. Analysis of the FVIII transcript of the haemophilic dogs revealed a novel sequence, termed ch8, after exon 22 of the FVIII transcript.
Ch8 consisted of a 421 nucleotide sequence followed by a polyadenylation signal sequence and a polyA tail, and contained several stop codons. Analysis of the genomic DNA of healthy dogs revealed that ch8 is also located upstream of the FVIII gene. The presence of an upstream DNA element downstream of exon 22 in the FVIII transcript of haemophilic dogs suggested a possible intron 22 inversion of the canine FVIII gene (Lozier et al., 2002).
Mogą zainteresować Cię również
POSTĘPOWANIA
w weterynarii