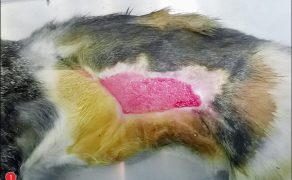
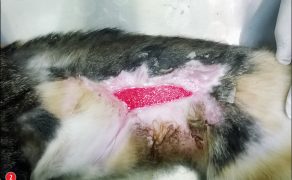
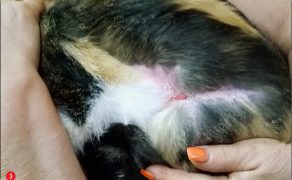
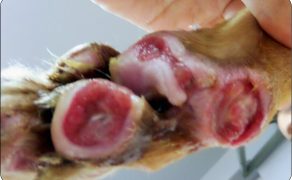
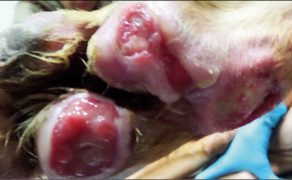
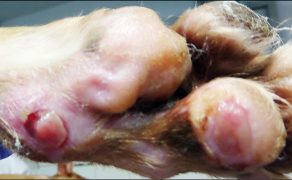
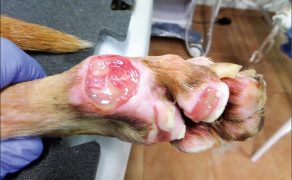
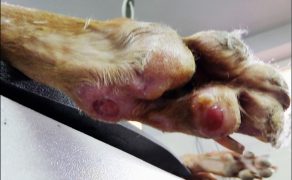
Zastosowanie czynników wzrostu w gojeniu dużych ubytków skórnych i ran przewlekłych u zwierząt towarzyszących
Piśmiennictwo
- Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Tomic-Canic M.: Growth factors and cytokines in wound healing. „Wound Repair Regen.”, 16 (2008), 585-601.
- Freedberg I.M., Tomic-Canic M., Komine M., Blumenberg M.: Keratins and the keratinocyte activation cycle. „J Invest Dermatol”, 2001; 116: 633-40.
- Hantash B.M., Zhao L., Knowles J.A., Lorenz H.P.: Adult and fetal wound healing. „Front Biosci”, 2008; 13: 51-61.
- Martin P.: Wound healing – aiming for perfect skin regeneration. „Science”, 199; 276: 75-81.
- Santoro M.M., Gaudino G.: Cellular and molecular facets of keratinocyte reepithelization during wound healing. „Exp. Cell Res.”, 2005; 304: 274-286.
- Atala A., Lanza R., Thomson J.A., Nerem R.: Principles of Regenerative Medicine. Elsevier, New York 2011.
- Fan Q., Yee C.L., Ohyama M., Tock C., Zhang G., Darling T.N., Vogel J.C.: Bone marrow-derived keratinocytes are not detected in normal skin and only rarely detected in wounded skin in two different murine models. „Exp. Hematol.”, 2006; 34: 672-679.
- Werner S., Grose R.: Regulation of wound healing by growth factors and cytokines. „Physiol. Rev.”, 2003; 83: 835-870.
- Lanza R., Langer R., Vacanti J.: Principles of Tissue Engineering. Elsevier, 2000.
- Pierce G.F., Tarpley J.E., Tseng J., Bready J., Chang D., Kenney W.C., Rudolph R., Robson M.C., Vande Berg J., Reid P.: Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic non-healing wounds. „J. Clin. Invest.”, 1995; 96: 1336-1350.
- Jinnin M., Ihn H., Mimura Y., Asano Y., Yamane K., Tamaki K.: Regulation of fibrogenic/fibrolytic genes by platelet-derived growth factor C, a novel growth factor, in human dermal fibroblasts. ”J Cell Physiol”, 2005; 202: 510.
- Mast B.A., Schultz G.S.: Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. „Wound Repair Regen”, 1996; 4: 411-20.
- Brown G.L., Curtsinger L., Brightwell J.R., Ackerman D.M., Tobin G.R., Polk H.C. Jr., George-Nascimento C., Valenzuela P., Schultz G.S.: Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. „J Exp Med” 1986; 163: 1319-24.
- Brown G.L., Curtsinger L.J., White M., Mitchell R.O., Pietsch J. ,Nordquist R., von Fraunhofer A., Schultz G.S.: Acceleration of tensile strength of incisions treated with EGF and TGF-beta. „Ann Surg”, 1988; 208: 788-94.
- Kim I., Mogford J.E., Chao J.D., Mustoe T.A.: Wound epithelialization deficits in the transforming growth factor alpha knockout mouse. „Wound Repair Regen”, 2001; 9: 386-90.
- Wood L.C., Elias P.M., Calhoun C., Tsai J.C., Grunfeld C., Feingold K.R:. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. „J Invest Dermatol”, 1996; 106: 397-403.
- Ornitz D.M.: FGFs, heparan sulfate and FGFRs: complex interactions essential for development. „Bioessays”, 2000; 22: 108-12.
- Powers C.J., McLeskey S.W., Wellstein A.: Fibroblast growth factors, their receptors and signaling. „Endocr Relat Cancer”, 2000; 7: 165-97.
- Sogabe Y., Abe M., Yokoyama Y., Ishikawa O.: Basic fibroblast growth factor stimulates human keratinocyte motility by Rac activation. „Wound Repair Regen”, 2006; 14: 457-62.
- Sasaki T.: The effects of basic fibroblast growth factor and doxorubicin on cultured human skin fibroblasts: relevance to wound healing. „J Dermatol”, 1992; 19: 664-6.
- Robson M.C.: The role of growth factors in the healing of chronic wounds. „Wound Repair Regen”, 1997; 5: 12-7.
- Robson M.C., Phillips L.G., Lawrence W.T., Bishop J.B., Youngerman J.S., Hayward P.G., Broemeling L.D., Heggers J.P.: The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. „Ann Surg”, 1992; 216: 401-408.
- Friedlander M., Brooks P.C., Shaffer R.W., Kincaid C.M., Varner J.A., Cheresh D.A.: Definition of 2 angiogenic pathways by distinct alpha(V) integrins. „Science”, 1995; 270: 1500-1502.
- Clark RAF: The molecular and cellular biology of wound repair. 2nd ed. New York: Plenum Press, 1996.
- Riedel K., Riedel F., Goessler U.R., Germann G., Sauerbier M.: Tgf-beta antisense therapy increases angiogenic potential in human keratinocytes in vitro. „Arch Med Res”, 2007; 38: 45-51.
- White L.A., Mitchell T.I., Brinckerhoff C.E.: Transforming growth factor beta inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. „Biochim Biophys Acta”, 2000; 1490: 259-68.
- Cordeiro M.F., Reichel M.B., Gay J.A., D’Esposita F., Alexander R.A., Khaw P.T.: Transforming growth factor-beta1,-beta2, and -beta3 in vivo: effects on normal and mitomycin C-modulated conjunctival scarring. „Invest Ophthalmol VisSci” 1999; 40: 1975-82.
- Shah M., Foreman D.M., Ferguson M.W.: Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. „J Cell Sci”, 1995; 108 (Pt 3): 985-1002.
- Tyrone J.W., Marcus J.R., Bonomo S.R., Mogford J.E., Xia Y,, Mustoe T.A.: Transforming growth factor beta3 promotes fascial wound healing in a new animal model. „Arch Surg”, 2000; 135: 1154-9.
- Bhansali A., Venkatesh S., Dutta P., Dhillon M.S., Das S., Agrawal A.: Which is the better option: recombinant human PDGF-BB 0.01% gel or standard wound care, in diabetic neuropathic large plantar ulcers off-loaded by a customized contact cast? „Diabetes Res. Clin. Pract.”, 2009; 83: 13-16.
- Hong J.P., Jung H.D., Kim Y.W.: Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. „Ann. Plast. Surg.”, 2006; 56: 394-398.
- Roubelakis M.G., Trohatou O., Roubelakis A., Mili E., Kalaitzopoulos I., Papazoglou G., Pappa K.I., Anagnou N.P.: Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. „Stem Cell Rev.”, 2014; 10: 417-428.
- Atala A., Lanza R., Thomson J.A., Nerem R.: Principles of Regenerative Medicine. Elsevier, New York 2011.
- White L.A., Mitchell T.I., Brinckerhoff C.E.: Transforming growth factor beta inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. „Biochim Biophys Acta”, 2000; 1490: 259-68.
- Chen S.M., Ward S.I., Olutoye O.O., Diegelmann R.F., Kelman Cohen I.: Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. „Wound Repair Regen”, 1997; 5: 23-32.
- Bernabei R., Landi F., Bonini S., Onder G., Lambiase A., Pola R., Aloe L.: Effect of topical application of nerve-growth factor on pressure ulcers. „Lancet”, 1999; 354: 307.
- Lewin G.R., Mendell L.M: Nerve growth factor and nociception. „Trends Neurosci”, 16: 353-359, 1993.
- Mann A., Breuhahn K., Schirmacher P., Blessing M:. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: stimulation of keratinocyte proliferation, granulation tissue formation and vascularization. „J Invest Dermatol”, 2001; 117: 1382-90.
- Gough A., Clapperton M., Rolando N., Foster A..V, Philpott- Howard J., Edmonds M.E.: Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. „Lancet”, 1997; 350: 855-9.
- Bussolino F., Wang J.M., Defilippi P., Turrini F., Sanavio F., Edgell C.J., Aglietta M., Arese P., Mantovani A.: Granulocyteand granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. „Nature”, 1989; 337: 471-3.
lek. wet. Aneta Bocheńska
lek. wet. Anna Kaniewska
dr n. wet. Roman Aleksiewicz
dr n. wet. Grzegorz Ramisz
lek. wet. Maciej Kiełbowicz
lek. wet. Radosław Owczarek
Galeria
Mogą zainteresować Cię również
110
ALGORYTMY
POSTĘPOWANIA
w weterynarii
POSTĘPOWANIA
w weterynarii